Overview
If you think about bacteria, what comes to mind? Some might say that bacteria are bad organisms that cause infectious diseases, others may say that fermented soy beans (Natto) are made using bacteria. These are true statements, but bacteria are actually very useful in modern life science technology. Such a small organism can move by itself (motility), and can sense environmental information (recognition). Bacteria have the ability to move toward better environments (taxis). Our research interests focus on energy transduction and sensory transduction in bacteria. A special emphasis is put on the supramolecular complexes located in the cytoplasmic membrane, namely the flagellar motor and the photoreceptor-kinase complex. The bacterial flagellar motor is an elaborate molecular machine driven by ion (proton or sodium ion) motive force. We want to understand how electrochemical energy (i.e. ion flux) is converted to mechanical energy (i.e. torque) to drive this tiny rotary motor. We also want to know how the shape and function of the biological membrane are regulated using giant liposomes.Human neurons deteriorate over the span of decades. Furthermore, we have been studying the genetic mechanisms that underlie the aging of neuronal functions and the environmental factors that affect the process using the nematode C. elegans. as a model organism. Our group focus on the microbial motility and sensing and studied them by the aspects of system biology by use of structural biology, nanotechnology, molecular biology, molecular genetics, biophysics, and so on. |
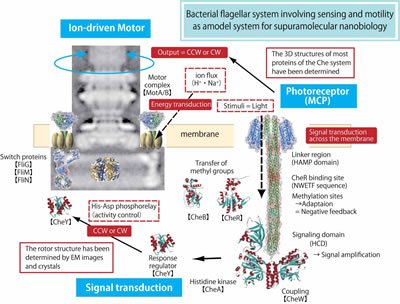
Mechanism of energy coupling in the flagellar motor
The bacterial flagellum is a helical filament driven by a reversible rotary motor at its base. The energy source for the motor is an electrochemical proton (or sodium ion) gradient across the cytoplasmic membrane. The flagellar filament is a huge organelle, whose length is several times larger than the cell body. This corresponds to the case that one who is 160 cm tall swings a string of 4 m long at 1,700 rpm, that speed is close equivalent to the F1 car engine! This flagellar system is the only rotary locomotive organelle to be described. However, the mechanism underlying flagellar motor rotation has yet to be fully elucidated. Therefore, we are trying to clarify the energy coupling using the following approaches: (i) Transform proton-driven motors into sodium-driven motors by genetic manipulation, (ii) Precisely measure flagellar rotation using a tiny bead attached to the rotating filament, (iii) Investigate behavior of the stator proteins on the membrane by using GFP-fusion constructs, (iv) Determine the protein structure of the motor, and (v) Reconstitute the entire motor in a proteoliposome using purified motor components. With these projects, we wish to elucidate the rotation mechanism of this magnificent molecular machine. |
Regulation of the number and placement of the polar flagellum in Vibrio
Precise regulation of the number and placement of flagella is critical for the mono-flagellated bacterium Vibrio alginolyticus to swim efficiently. We have previously proposed a model in which a putative GTPase of FlhF localization at the pole determines the polar location and generation of a flagellum, a putative ATPase FlhG interacts with FlhF to prevent FlhF from localizing at the pole, and thus FlhG negatively regulates the flagellar number in V. alginolyticus cells. By clarifing the function of FlhF and FlhG, we would like to know how to precisely regulate the number and placement of flagella. |
Mechanism underlying aging of neuronal functions
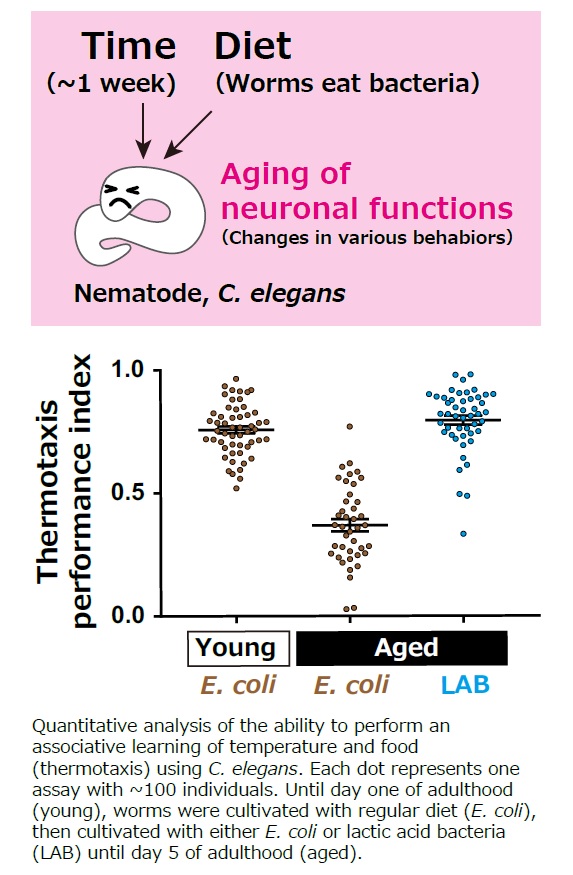
Cellular aging can manifest through the cease of cell division, which is proposed to be a result of the shortening of the chromosomes’ terminal structures (telomeres). However, telomere shortening does not affect neurons that have completed cell division. DNA damage, protein aggregation, and cell membrane damage can all cause the aging of postmitotic cells. However, while the passive accumulation of these damages can explain the acceleration of aging, it does not fully explain the varying rates of aging among different species. Thus, our goal is to unravel the mechanism behind the deterioration of neuronal function with an additional focus on diet as a critical environmental factor. |
References
|
■Bacterial group |